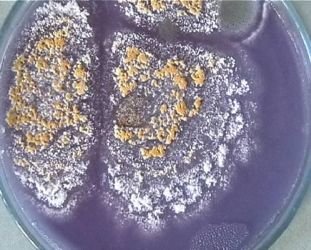
Malbranchea cinnamomea grown on nutrient agar.
Synonyms
Geotrichum cinnamomeum (Lib.) Sacc.
Malbranchea pulchella var. sulfurea (Miehe) Cooney & R. Emers.
Malbranchea sulfurea (Miehe) Sigler & J.W. Carmich.
Malbranchea sulfurea (Miehe) Pidopl. [as ‘sulphurea‘]
Thermoidium sulphureum Miehe [as ‘sulfureum‘]
Basionym
Trichothecium cinnamomeum Lib.
Description
Malbranchea cinnamomea is a thermophilic fungi, having a minimum temperature of growth at or above 20°C. This ascomycete fungi is often isolated from higher-temperature environments such as animal hair, or sun-heated desert soils.

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Medicinal properties
Antibiotic activity
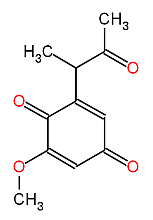
M. cinnamomea produces a quinone antibiotic (6-(1-acetylethyl)-2-methoxy-2,5-cyclohexadiene-1,4-dione) named malbranicin (Chiung et al., 1993), shown in figure 1.
Malbranicin has antimicrobial activities against Gram-positive bacteria. The antimicrobial spectrum is given in table 1.
|
Microorganism
|
MIC (µg/ml)
|
|
Staphylococcus aureus
|
25
|
|
S. epidermidis
|
100
|
|
S. xylosus
|
100
|
|
Bacillus subtilis
|
25
|
|
Micrococcus luteus
|
100
|
|
M. roseus
|
6.25
|
|
Enterococcus faecalis
|
>100
|
|
Escherichia coli
|
>100
|
|
Serratia marscecens
|
>100
|
|
Pseudomonas aeruginosa
|
>100
|
|
Candida albicans
|
>200
|
|
Saccharomyces cerevisiae
|
>200
|
|
Aspergillus niger
|
>200
|
|
Fusarium moxysporum
|
200
|
|
Penicillium chrysogenum
|
>200
|
Table 1. Antimicrobial spectrum of malbranicin, as reported by Chiung et al., 1993. Minimal inhibitory concentration (MIC) was determined using the serial diffusion assay method.
Additionally, the authors report that malbranicin was cytotoxic at 0.7 µg/ml against P388 cells and 2.8 µg/ml to KB cells.
A stereoselective synthesis of (S)-(+)-malbranicin has been reported (Vanderlei et al., 1997).
Other compounds isolated from this fungus include dihydromalbranicin, various novel substituted quinones, including hydroquinone. The compound 2-(7-methoxymalbranicin) at a concentration of 42 µm inhibited by 67% Tax/CREB-mediated expression of β-galactosidase in a recombinant strain of Saccharomyces cerevisiae (Schegel et al., 2003a).
A compound, 7-methoxy-2,3-dimethylbenzofuran-5-ol, isolated from M. cinnamomea, was reported to have antioxidant activity (Schlegel et al., 2003b).
- see the Japan Society for Culture Collections (JSCC) for a culture source
- see here for details about a patent on polysaccharide extraction from mycelial cultures of this and similar specie
Chiung YM, Fujita T, Nakagawa M, Nozaki H, Chen GY, Chen ZC, Nakayama M.
A novel quinone antibiotic from Malbranchea cinnamomea TAIM 13T54.
J Antibiot (Tokyo). 1993 46(12):1819-26.
McKay DJ, Stevenson KJ.
Lipoamide dehydrogenase (E. C. 1.6.4.3) from Malbranchea pulchella var. sulfurea: isolation and characterization.
Biochemistry 1979 18:4702-7.
Schlegel B, Hanel F, Gollmick FA, Saluz HP, Grafe U.
New quinones and hydroquinones from Malbranchea cinnamomea HKI 286 and HKI 296 and interaction with Tax/CREB expression system in yeast.
J Antibiot (Tokyo). 2003a 56(11):917-22.
Schlegel B, Hartl A, Gollmick FA, Grafe U.
7-methoxy-2,3-dimethylbenzofuran-5-ol, a new antioxidant from Malbranchea cinnamomea HKI 0286.
J Antibiot (Tokyo). 2003b 56(9):792-4.
Vanderlei JML, Coelho F, Almeida WP.
A stereoselective synthesis of malbranicin.
Tetrahedron Asymmetry. 1997 8(16): 2781-5.