The medicinal mushroom Ampulloclitocybe clavipes
The club-footed clitocybe, Ampulloclitocybe clavipes (Pers.) Redhead.
Classification
Kingdom Fungi
Phylum Basidiomycota
Class Basidiomycetes
Order Agaricales
Family Tricholomataceae
Genus Clitocybe
Synonyms
Agaricus clavipes Pers.
Syn. meth. fung. (Göttingen) 2: 353 (1801)
Clavicybe clavipes (Pers.) Harmaja
Karstenia 42(2): 42 (2002)
Clitocybe clavipes (Pers.) P. Kumm.
Führ. Pilzk. (Zwickau): 124 (1871)
Common names
Club-footed clitocybe
Club foot
Clavate-stalked clitocybe
Clitocybe à pied en massue (French)
Hoteishimeji (Japanese)
Keulen-Trichterling (German)
Knotsvoettrechterzwam (Dutch)

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Description
Cap: 3-7 cm, applanate to slightly depressed, often slightly umbonate, grayish-brown to dark brown, lighter in color towards the margin, smooth to minutely subtomentose, somewhat greasy when moist, marginal zone somewhat decurved with an involute margin.
Gills: deeply decurrent, thin, crowded to subdistant, 3-6 mm broad, segmentiform, whitish, soon pale-cream to lemon-yellow, with entire, concolorous edge.
Stem: 3-6cm tall x 0.5-1.4 cm diameter, usually clavate towards base, but sometimes cylindrical to subclavate, stuffed, pale greyish cream, slightly to rather coarsely fibrillose–striate; context whitish, spongy in base of stem.
Smell: pleasant, sweet – similar to Iris flowers, even more so in dried specimens.
Taste: mild, indistinct.
Spore print: white.
Spores: sub-spherical to ellipsoid, smooth, hyaline, non-amyloid, with droplets, 6.0-9.5 x 4.0-5.0 µm.
Habitat: alone, in groups or gregariously under deciduous and coniferous trees (especially pine and spruce); common, widespread in the Northern hemisphere; summer-fall.
Edibility: some guide books say ‘edible with caution’. However, this mushroom is toxic in combination with alcohol due to presence of Type 6 toxins, similar to disulfram (Cochran and Cochran, 1978). [see also Coprinus atramentaria for a similar reaction caused by coprine] Furthermore, a toxicity text of this species showed that the crude extract was fatally toxic to albino mice 4 hours after consumption (Natour et al., 1993).
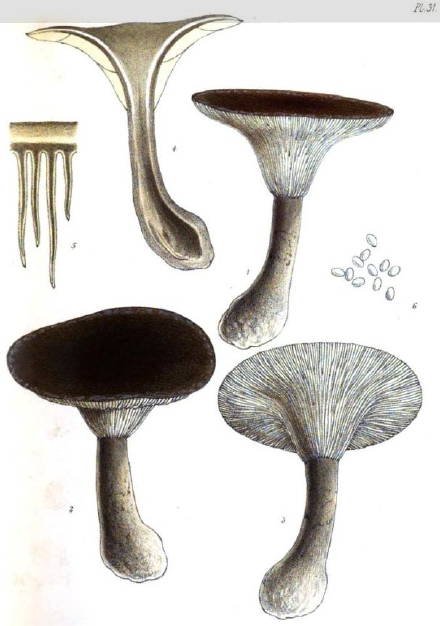
Drawings of Ampulloclitocybe (formerly Agaricus) clavipes from Saunders et al., 1871, plate 31.
Fatty acid composition
GC and GC-MS analysis of the fatty acid methyl esters of Ampulloclitocybe clavipes revealed forty one fatty acids (FA). The mixtures comprise straight chained saturated FA with odd and even chain lengths in the range 10 to 26 carbon atoms, branched chained saturated FA with odd and even chain lengths from C13 to C17, straight chained monoenoic FA with odd and even chains ranging from C16 to C24, C16 and C18 straight chained dienoic FA and trienoic FA. Linoleic, oleic and palmitic acids accounted for 86.2% of the total FA content. In the dienoic fatty acid fraction, A. clavipes contains a series of dienoic isomers as minor components and traces: 11,14-18:2 (0.3%), 7,10-16:2, 7,10-18:2, 9,11-18:2 and 10,12-18:2 (0.1% each) and four positionally isomeric 18:2 FA with unknown positions of the double bonds (Marekov et al., 2007).
Bioactive compounds
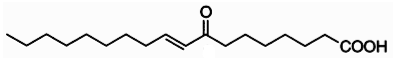
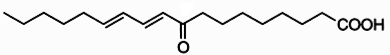
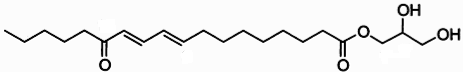
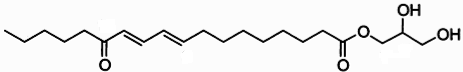
Clavilactones A-C (shown below) have been isolated from cultures of A. clavipes (Arnone et al., 1994).
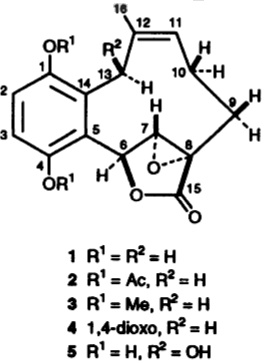
Clavilactones D and E were later isolated from agar cultures (Cassinelli et al., 2000).
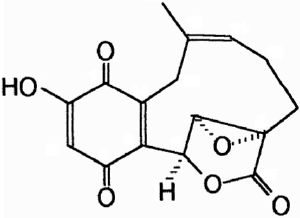
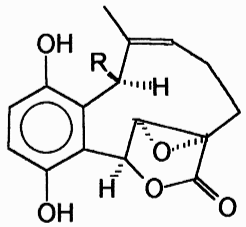
Clavilactone D potently inhibited epidermal growth factor receptor (EGFR) (IC50 = 5.5 µM) in a kinase assay (Merlini et al., 2000). The clavilactones represent a novel class of tyrosine kinase inhibitory activity and may hold promise as an anti-tumor therapy (Cassinelli et al., 2000).
Medicinal Properties
Antibacterial activity
Clavilactone B had antibacterial activity against Bacillus subtilis, B. cereus, Sarcina lutea (50 µg/disc); clavilactone A was only active against B. subtilis at 100 µg/disc (Arnone et al., 1994).
All clavilactones had antifungal activity, as determined using bioautography on Cladosporium cladosporioides and C. cucumerinum with amounts as low as 50 µg per plate (Arnone et al., 1994).
Antitumor activity
Polysaccharides extracted from the mycelial culture of A. clavipes and administered intraperitoneally into white mice at a dosage of 300 mg/kg inhibited the growth of Sarcoma 180 and Ehrlich solid cancers by 70% and 60%, respectively (Ohtsuka et al., 1973).
References
Arnone A, Cardillo R, Meille SV, Nasini G, Tolazzi M.
Secondary mold metabolites. 47. Isolation and structure elucidation of clavilactones AC, new metabolites from the fungus Clitocybe clavipes.
J Chem Soc-Perk Trans 1. 1994 15: 2165-8.
Bousset M.
Sur la presence d’acide cyanhydrique chez Clitocybe clavipes et Rhodopaxillus nudus.
Bull Mens Soc Linn Lyon. 1941 10(10):154-5.
Cassinelli G, Lanzi C, Pensa T, Gambetta RA, Nasini G, Cuccuru G, Cassinis M, Pratesi G, Polizzi D, Tortoreto M, Zunino F.
Clavilactones, a novel class of tyrosine kinase inhibitors of fungal origin.
Biochem Pharmacol. 2000 59(12):1539-47.
Cochran KW, Cochran MW.
Clitocybe clavipes – Antabuse-like reaction to alcohol.
Mycologia. 1978 70(5):1124-5.
Jarvis MC, Miller AM, Sheahan J, Ploetz K, Ploetz J, Watson RR, Palma Ruiz M, Pascario V, Carlos A, Garcia Alvarado J, Lopez Ramirez A, Orr B.
Edible wild mushrooms of the Cofre de Perote region, Veracruz, Mexico: An ethnomycological study of common names and uses.
Economic Botany. 2004 58(Suppl. S):S111-S115.
Kawagishi H, Miyazawa T, Kume H, Arimoto Y, Inakuma T.
Aldehyde dehydrogenase inhibitors from the mushroom Clitocybe clavipes.
J Nat Prod. 2002 65(11):1712-4.
Marekov I, Antonova D, Gyosheva M, Momchilova S, Nikolova-Damyanova B.
Fatty acid composition of lipids from two macrofungal species belonging to genus Clitocybe.
CR Acad Bulg Sci. 2007 60(7):757-762.
Merlini L, Nasini G, Scaglioni L, Cassinelli G, Lanzi C.
Structure elucidation of clavilactone D: an inhibitor of protein tyrosine kinases.
Phytochem. 2000 53(8):1039-41.
Natour RM, Salhab AS, El-Moumani AR, Saba EF.
Wild mushroom in Jordan.
Dirasat Series B Pure and Applied Sciences. 1993 19(2):47-60.
Noordeloos ME, Boekhout T, Vellinga EC, Arnolds EJM. 1995.
Flora agaricina Neerlandica; critical monographs on families of agarics and boleti occurring in the Netherlands, v.3.
CRC Press
Google Books
Ohtsuka S, Ueno S, Yoshikumi C, Hirose F, Ohmura Y, Wada T, Fujii T, Takahashi E.
Polysaccharides having an anticarcinogenic effect and a method of producing them from species of Basidiomycetes.
UK Patent 1331513, 26 September 1973.
Pemberton RT.
Agglutinins (lectins) from some British higher fungi.
Mycol Res. 1994 98(3):277-90.
Redhead SA, Lutzoni F, Moncalvo JM, Vilgalys R.
Phylogeny of Agarics: partial systematics solutions for core omphalinoid genera in the Agaricales (Euagarics).
Mycotaxon 2002 83:19-57.
Saunders WW, Smith WG, Bennett AW. 1871.
Mycological Illustrations: Being Figures and Descriptions of New and Rare Hymenomycetous Fungi.
London: John van Voorst.
Digitized in Google Books
Yamaura Y, Fukuhara M, Kawamata S, Satsumabayashi H, Takabatake E, Hashimoto T.
Effects of Clitocybe clavipes extract on the components and enzymes related to ethanol metabolism in mice.
J Food Hyg Soc Jpn. 1986 27(5):522-7.
Yamaura Y.
Classification of poisonous mushrooms according to their biochemical effects in mice.
Nippon Eiseigaku Zasshi-Japanese Journal of Hygiene. 1988 43(2):669-78.



