The rooting shank, Xerula radicata (Relhan) Dörfelt.
Synonyms
Agaricus radicatus Relhan
Fl. cantab., Suppl.: 28 (1786)
Collybia radicans P. Kumm.
Führer Pilzk. (Zwickau): 117 (1871)
Collybia radicata (Relhan) Quél.
Mém. Soc. Émul. Montbéliard, Sér. 2 5: 92 (1871)
Gymnopus radicatus (Relhan) Gray
Nat. Arr. Brit. Pl. (London) 1: 605 (1821)
Mucidula radicata (Relhan) Boursier
Bull. trimest. Soc. mycol. Fr. 40: 332 (1924)
Mucidula radicata f. marginata Konrad & Maubl.
Flore d’Algerie, Cryptogamie 2: pl. 199 (1931)
Oudemansiella radicata (Relhan) Singer
Annls mycol. 34(4/5): 333 (1936)
Oudemansiella radicata var. marginata (Konrad & Maubl.) Bon & Dennis
in Bon, Docums Mycol. 15(no. 59): 51 (1985)
Xerula radicata var. marginata (Konrad & Maubl.) ined.
Common names
Rooting shank
Beech rooter
Description
Cap: 3-10 cm diameter, yellow or olive-brown; convex or campanulate, in age becoming plano-convex with broad umbo; sulcate, surface viscid but drying shiny, sometimes radially wrinkled. Flesh pallid, otherwise concolorous and thin. Margin initially incurved, later uplifted.
Gills: white, adnexed or notched, thick, broad, distant.
Stem: 5-20 cm tall x 0.3-1 cm diameter; white at the apex, becoming tinged cap color below, slender, tapering upwards, deeply rooting; ring absent; flesh white and firm.
Spores: hyaline, smooth, ellipsoid, non-amyloid, with droplets, 12-16 x 9-12 µm. See Peterson (2008) for some detailed views of various Xerula spores using electron microscopy.
Spore print: white
Habitat: typically solitary or trooping; under or near deciduous trees, especially beech, attached to roots or buried wood.
Taste: mild.
Edibility: edible.

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Fatty acid composition
The lipid composition of this mushroom has been analyzed (Yilmaz et al., 2006). Of note, the mushroom caps contain 42.7% polyunsaturated fatty acids (percentage of total fatty acids), most of which is linoleic acid, an essential fatty acid in humans.
Medicinal properties
Antihypertensive effects
The molecule oudenone is a fungal metabolite that was first isolated from the culture filtrate of X. radicata (as Oudemansiella radicata) (Umezawa et al., 1970). Shortly after, the structure and synthesis of oudenone were reported (Ohno et al., 1971). Oudenone is a strong inhibitor of catecholamine biosynthesis – specifically, it inhibits the enzymes phenylalanine and tyrosine hydroxylase. The physiological effect of this enzyme inhibition is the reduction of blood pressure, the inhibition of tyrosine hydroxylase in the adrenal glands in vivo, as well as the reduction of tissue catecholamine levels in the adrenal glands, heart and brain in spontaneously hypertensive rats (Nagatsu et al., 1971). The biosynthesis and cyclization mechanism of oudenone have been elaborated using feeding experiments using 13C and 2H labeled precursors and NMR analysis (Tsantrizos et al., 1995; Tsantrizos et al., 1999). Various synthetic schemes have been proposed for the molecule (Tsujikawa and Hayashi, 1977; Bates and Farina, 1985; Flynn et al., 1995).
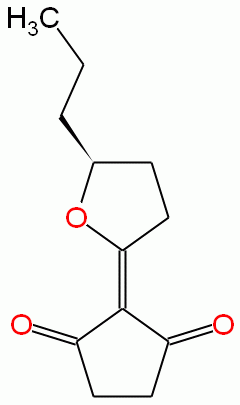
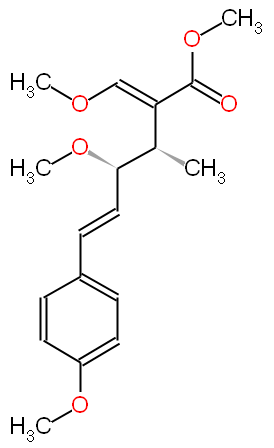
X. radicata has been shown to contain an antibiotic named oudemansin X (shown below), which lacked antibacterial activity against various organisms tested, but showed good antifungal activity (Anke et al., 1990). Later synthetic work described the chiral total synthesis of three kinds of oudemansin X, (-)-1, (+)-1 and (+)-1; like the parent molecule, the synthetic oudemansin X (-)-1 had strong antifungal activity against several molds and yeasts. (Umezawa et al., 1995). The lack of antibacterial activity corroborates the findings of Bianco et al. (1996).
Antitumor effects
Polysaccharides extracted from the mycelial culture of X. radicata and administered intraperitoneally into white mice at a dosage of 300 mg/kg inhibited the growth of Sarcoma 180 and Ehrlich solid cancers by 100% and 90%, respectively (Ohtsuka et al., 1973).
Web
Mushroom Expert
BioPix
Patent for the production and preparation of oudenone
References
Anke T, Werle A, Bross M, Steglich W.
Antibiotics from basidiomycetes. XXXIII. Oudemansin X, a new antifungal E-β-methoxyacrylate from Oudemansiella radicata (Relhan ex Fr.) Sing.
J Antibiot (Tokyo). 1990 43(8):1010-1.
Bates HA, Farina J.
Oxonium ion electrophiles – synthesis of the hypotensive oudenone.
J Org Chem. 1985 50(20):3843-5.
Bianco C, Ausilia M, Giardino L.
Antibiotic activity in Basidiomycetes. X. Antibiotic activity of mycelia and cultural filtrates of 25 new strains.
Allionia (Turin). 1996 34(0):39-43.
Dörfelt H.
The carpophore development of Xerula radicata (Basidiomycetes, Agaricales)
Flora 1982 172(6):533-61.
Flynn BL, Silveira CC, Demeijere A.
Selective formation of 4-ethoxy-5-methylene-2-cyclopentenones and 3-ethoxy-2-(1′-morpholinoalkenyl)-2-cyclopentenones from (1-ethoxy-3-morpholino-alkenylidene)pentacarbonylchromium complexes and terminal alkynes – a short enantioselective synthesis of the hypotensive oudenone.
Synlett. 1995 (8):812-4.
Koide T, Ozawa H.
Pharmacological analysis upon blood-pressure response to oudenone.
Jap J Pharmacol. 1976 26:P149-P.
Koide T, Ozawa H.
Pharmacological studies on blood-pressure response of oudenone, a tyrosine-hydroxylase inhibitor.
Folia Pharmacologica Japonica. 1976 72(3):P8-P9.
Koizumi S, Nagatsu T, Iinuma H, Ohno M, Takeuchi T, Umezawa H.
Inhibition of phenylalanine-hydroxylase, a pterin-requiring Mono-oxygenase, by oudenone and its derivatives.
Journal of Antibiotics. 1982 35(4):458-62.
Nagatsu T, Nagatsu I, Umezawa H, Takeuchi T.
Effect of oudenone on adrenal tyrosine hydroxylase activity in vivo and on tissue catecholamine concentrations.
Biochem Pharmacol. 1971 20(9):2505-7.
Ohtsuka S, Ueno S, Yoshikumi C, Hirose F, Ohmura Y, Wada T, Fujii T, Takahashi E.
Polysaccharides having an anticarcinogenic effect and a method of producing them from species of Basidiomycetes.
UK Patent 1331513, 26 September 1973.
Petersen RH, Methven AS.
Mating systems in the Xerulaceae – xerula.
Can J Bot-Revue Canadienne de Botanique 1994 72(8):1151-63.
Peterson RH.
Scanning electron microscope images of basidiospores of Xerula (Physalacriaceae, Agaricales).
Mycoscience. 2008 49:19-34.
PDF available from publisher
Ohno M, Okamoto M, Kawabe N, Umezawa H, Takeuchi T, Iinuma H, Takahash S.
Oudenone, a novel tyrosine hydroxylase inhibitor from microbial origin.
J Am Chem Soc. 1971 93(5):1285-&.
Ozawa H, Koide T.
Pharmacodynamic actions of (S)-2-[4,5-dihydro-5-propyl-2 (3h)-furylidene]-1,3-cyclopentanedione (oudenone).
Japanese Journal of Pharmacology. 1976 26(5):581-92.
Redhead SA, Ginns J, Shoemaker RA.
The Xerula (Collybia, Oudemansiella) radicata complex in Canada.
Mycotaxon 1997 30:357-405.
Sawada M, Iinuma H, Ohno M, Takeuchi T, Umezawa H, Nagatsu T.
Kinetic-analysis of the inhibition of tryptophan-hydroxylase, a pteridine-requiring monooxygenase, by oudenone and its derivatives.
Biogenic Amines. 1984 1(2):171-8.
Sakai T, Iwata K, Utaka M, Takeda A.
A convenient synthesis of hypotensive (+/-)-oudenone.
Bull Chem Soc Japan. 1987 60(3):1161-2.
Semerdzieva M, Buchalo AS, Huebsch P, Zakordonec OA, Wassser SP, Musilek V.
Comparative study of cultures of four species of the genus Oudemansiella.
Folia Microbiologica. 1988 33(2):115-120.
Tsantrizos YS, Yang X, McClory A.
Studies on the biosynthesis of the fungal metabolite oudenone. 2. Synthesis and enzymatic cyclization of an α-diketone, open-chain precursor into oudenone in cultures of Oudemansiella radicata.
J Org Chem. 1999 64(18):6609-6614.
Tsantrizos YS, Zhou F, Famili P, Yang XS.
Biosynthesis of the hypotensive metabolite oudenone by Oudemansiella radicata.1. Intact incorporation of a tetraketide chain elongation intermediate.
J Org Chem. 1995 60(21):6922-9.
Tsujikawa T, Hayashi M.
Studies on heterocyclic-compounds .7. Syntheses of oudenone and its related compounds .2.
Chem Pharm Bull. 1977a 25(12):3147-54.
Tsujikawa T, Nakagawa Y, Tsukamura K, Masuda K.
Studies on heterocyclic-compounds .6. Syntheses of oudenone and its related compounds .1.
Chem Pharm Bull. 1977b 25(10):2775-8.w
Tsujikawa T, Nakagawa Y, Tsukamura K, Masuda K.
Synthesis of oudenone and its related compounds.
Heterocycles. 1977c 6(3):261-6.
Umezawa H, Takeuchi T, Iinuma H, Suzuki K, Ito M, Matsuzak M.
A new microbial product, oudenone, inhibiting tyrosine hydroxylase.
J Antibio. 1970 23(10):514-&.
Umezawa I, Nozawa M, Nagumo S, Akita H.
Total synthesis of (racemic)-, (-)-, and (+)-oudemansin X.
Chem Pharm Bull. 1995 43(7):1111-8.
Yilmaz N, Solmaz M, Turkekul I, Elmastas M.
Fatty acid composition in some wild edible mushrooms growing in the middle Black Sea region of Turkey.
Food Chem. 2006 99(1):168-74.
Zou X.
Optimization of nutritional factors for exopolysaccharide production by submerged cultivation of the medicinal mushroom Oudemansiella radicata.
World J Microbiol Biotech. 2005 21(6-7):1267-71.




Do you have a culture of this strain?