The wood woollyfoot, Gymnopus peronatus (Bolton) Antonín, Halling & Noordel.
Classification
Kingdom Fungi
Phylum Basidiomycota
Class Basidiomycetes
Order Agaricales
Family Tricholomataceae
Genus Gymnopus
Synonyms
Agaricus peronatus Bolton
Hist. fung. Halifax 2: 58 (1788)
Agaricus urens Bull.
Herbier de la France 11: tab. 528, fig. 1 (1791)
Collybia peronata (Bolton) P. Kumm.
Führer Pilzk. (Zwickau): 116 (1871)
Collybia urens (Bull.) P. Kumm.
Führer Pilzk. (Zwickau): 115 (1871)
Marasmius peronatus (Bolton) Fr.
Anteckn. Sver. Ätl. Svamp.: 52 (1836)
Marasmius urens (Bull.) Fr.
Anteckn. Sver. Ätl. Svamp.: 52 (1836)
Common name
Wood woollyfoot

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Description
Cap: 2.5-6 cm diameter, convex to plane, often with broad umbo and irregularly lobed margins; glabrous; sometimes grooved above gills; yellow-brown to pink-brown with paler margin; hygrophanous; context white to pale yellow.
Stem: 3-7.5 cm long and 0.3-0.8 cm thick; smooth or floccose at apex becoming fibrillose-striate below, usually expanded towards base; ochre-brown to yellowish, often more orange-brown towards the base which is covered with buff to yellowish hairs.
Gills: free, distant, sometimes forked near stem; pale or yellowish changing to brownish; edges entire or finely whitish granulose fringed.

Spores: 8.5-10 x 3-4 µm, smooth, elliptic to subcylindrical.
Odor: mild.
Taste: peppery to pungent/acrid.
Edibility: considered too acrid to eat. Furthermore, reports of the presence of the toxic compound hydrocyanic acid (Bach, 1948) indicate consumption be avoided.
Habitat: usually found in dense groups in forest litter, on conifer and hardwood debris.
Bioactive compounds
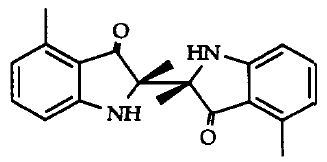
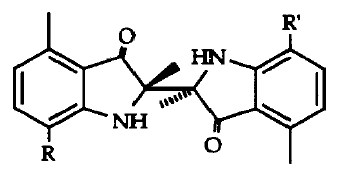
Two 3-indolinone dimers have been isolated from EtOAc extracts of the fruit bodies of Collybia peronata, peronatins A and B (shown below). The diastereoisomeric compounds are formed in the fruit bodies upon injury, from enzymatic hydrolysis of an indole precursor and oxidative coupling of the derived indoxyl radicals (Pang and Sterner, 1994). Further work on the synthesis and isomerization of these indole-compounds has been reported (Stachel et al., 1997).
The sesquiterpene deoxycollybolidol (3β-(3-furyl)-3,4,4aα,8β,9,9aα- hexahydro- 5-β-methyl-5,8-methano-lH-pyrano[3,4-d]oxepin- l,6(5H)-dione, shown below) was first identified from the chloroform-methanol extract of G. peronatus (Fogedal and Norberg, 1986), and its presence later confirmed (Castronovo et al., 2001).
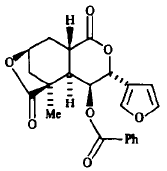
The sesquiterpene deoxycollybolidol, consisting of a tricyclic nucleus bearing both a γ- and a δ-lactone group, and a benzoate and a furan ring as substituents.
Medicinal properties
Antitumor effects
Reporting on the favorable influence of sodium citrate on the production of antibiotics in Marasmius spp (Öblom, 1950), it is suggested that this species has antibacterial activity: “Recent work on antibiotics has shown that Marasmius urens (Bull). Fr. is highly active against several pathogenic bacteria (Öblom and Wallmark, unpublished).” This work (i.e., the effect of organic acids on antibiotic production) was later elaborated and expanded (Öblom, 1951), and Staphylococcus aureus was used as the test bacteria.
Polysaccharides extracted from the mycelial culture of G. peronatus and administered intraperitoneally into white mice at a dosage of 300 mg/kg inhibited the growth of Sarcoma 180 and Ehrlich solid cancers by 60% (Ohtsuka et al., 1973).
Links
See a photo gallery at Biopix
References
Bach E.
Marasmius peronatus and Marasmius perforans form hydrocyanic acid.
Friesia. 1948 3(5):377-8.
Castronovo F, Clericuzio M, Toma L, Vidari G.
Fungal metabolites. Part 45: The sesquiterpenes of Collybia maculata and Collybia peronata.
Tetrahedron. 2001 57(14):2791-8.
Dix NJ, Simpson AP.
Decay of leaf litter by Collybia peronata.
Transactions of the British Mycological Society. 1984 83(AUG):37-41.
Fogedal M, Norberg T.
Deoxycollybolidol, a sesquiterpene from Collybia peronata.
Phytochemistry. 1986 25(11):2661-3.
Higham CA, Jones ERH, Keeping JW, Thaller V.
Natural Acetylenes .45. Polyacetylenes from cultures of fungus Collybia peronata (Bolt Ex Fr) Kummer.
J Chem Soc-Perkin Transactions 1. 1974 (16):1991-4.
Öblom K.
Effects of nutrition of Marasmius urens upon the production of antibiotic substances.
Nature 1950 166(4231): 950-1.
Öblom K.
Nutritional effects upon the antibiotic production of Marasmius urens (Bull) Fr .1. The influence of organic acids.
Physiologia Plantarum 1951 4(3): 563-73.
Ohtsuka S, Ueno S, Yoshikumi C, Hirose F, Ohmura Y, Wada T, Fujii T, Takahashi E.
Polysaccharides having an anticarcinogenic effect and a method of producing them from species of Basidiomycetes.
UK Patent 1331513, 26 September 1973.
Pang ZJ, Sterner O.
The isolation of 2,2′-biindoline-3,3′-diones from injured fruit bodies of Collybia peronata and Tricholoma scalpturatum.
J Nat Prod 1994 57(6):852-7.
Stachel SJ, Nilges M, VanVranken DL.
Synthesis and isomerization of biindolinones from Collybia peronata and Tricholoma scalpturatum.
J Org Chem. 1997 62(14):4756-62.
Tyler G.
Accumulation and exclusion of metals in Collybia peronata and Amanita rubescens.
Trans Br Mycol Soc. 1982 79(Oct):239-45.


