Looking like a charred, disembodied hand from an old B-movie, it’sXylaria polymorpha (Pers.) Grev.
Synonyms
Coelorhopalon obovatum (Berk.) Overeem
Hypoxylon polymorphum (Pers.) Mont.
Penzigia obovata (Berk.) Speg.
Sphaeria obovata Berk.
Sphaeria polymorpha Pers.
Xylaria corrugata Har. & Pat.
Xylaria obovata (Berk.) Berk.
Xylaria rugosa Sacc., (1906)
Xylosphaera obovata (Berk.) Dennis
Xylosphaera polymorpha (Pers.) Dumort.
Basionym
Sphaeria polymorpha Pers. 1797
Common name
Dead man’s fingers

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Description
In Indian traditional medicine (Ayurvedic medicine), the powdered fruit bodies, mixed in equal proportion with sugar, is used to promote lactation after birth (Rai et al., 1993).
This fungus is responsible for root rot, and the leaves of afflicted trees dry out and drop prematurely. According to the Canadian Forest Service website’s page, X. polymorpha is one of the few ascomycete species capable of decomposing the wood of sugar maple and box elder.
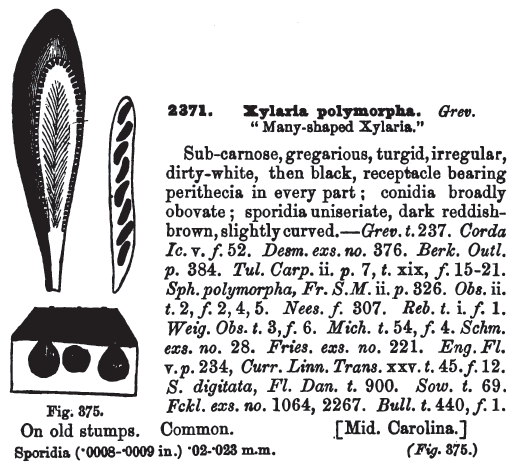
Fruit body: 3-8 cm tall, 1-3 cm wide, irregularly club-shaped (fingerlike), often knobby or gnarled, white to grayish and powdery initially (during the asexual stage), then black with a finely wrinkled or roughened or cracked surface.
Flesh: tough, white. The perithecia (spore-producing cavities) are just below the surface crust. The asci are 200 x 10 µm.
Spores: the ascospores are dark-brown to blackish, fusiform, smooth, 20-30 x 5-10 µm; the conidia are smaller, elongated or elliptical, smooth, and hyaline.
Habitat: gregarious on stumps and fallen timber of deciduous trees, including Acer rubrum, Coffea arabica, Platanus acerifolia and Shorea robusta (Sivanesan and Holliday, 1998). Season all year. Common. Because of the toughness of the species, the fruiting bodies can persist for a long time. In fact, one early paper (Barnes, 1940) reported finding a 16-year old specimen living on a piece of elm wood.
Edibility: Inedible.
Distribution: America and Europe.
Bioactive compounds
2-Hexylidene-3-methylsuccinic acid, aka piliformic acid, is the major metabolite produced by X. polymorpha (Anderson et al., 1985).
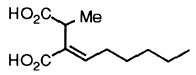
This compound (shown above), which was later isolated from the marine fungus Halorosellinia oceanica BCC 5149, showed moderate cytotoxicity against KB and BC-1 cell lines (Chinworrungsee et al., 2001).
Dead man’s fingers was shown to contain about 6% mannitol (dry weight), a sugar used as a diuretic agent (Snatzke and Wolff, 1987). Other compounds include 4-(3′-Acetyl-2′,6′-dihydroxy-5′-methylphenyl)- 4-hydroxy- 2-methoxybutanoic acid (globoscinic acid) and 5-(3′-acetyl-2′,6′-dihydroxy- 5′-methylphenyl)-3-methoxy- 2,3,4,5-terahydrofuran-2-one (globoscin) (Adeboya et al., 1995), and two cytotoxic cytochalasins 19,20-epoxycytochalasin Q and its deacetyl analog (Dagne et al., 1994). Of the latter two compounds, both were shown to be cytotoxic, but inactive in an HIV-protease inhibitory assay and a mechanism-based DNA damaging yeast assay.
Research has also gone into determining the optimal conditions for the production of X. polymorpha polysaccharides grown in liquid culture (Yang and Huaan, 2004).
Two new polypropionates designated as xylarinic acids A (4,6,8-trimethyl- 2,4-decadienoic acid) and B (2,4,6-trimethyl- 2-octenoic acid) were isolated from X. polymorpha fruiting bodies. Both compounds displayed significant antifungal activity against the pathogenic plant fungi Pythium ultinum, Magnaporthe grisea, Aspergillus niger, Alternaria panax, and Fusarium oxysporium, but they did not show any antibacterial nor cytotoxic effects (Jang et al., 2007).
Tom Volk’s fungus of the month, April 2000
Mushroom Expert
More pictures and description here at the Fungi on Wood pages
Another informative page from the Penn State New Kensington Virtual Nature Trail
References
Abate D, Abraham WR, Meyer H.
Cytochalasins and phytotoxins from the fungus Xylaria obovata.
Phytochem. 1997 44(8):1443-8.
Adeboya M, Edwards RL, Laessoe T, Maitland DJ, Whalley ASJ.
Metabolites of the higher fungi .28. Globoscinic acid and globoscin, a labile acid-lactone system from Xylaria globosa and Xylaria obovata.
J Chem Soc-Perk Trans. 1995 1(16):2067-72.
Abstract – RSC Publishing
Anderson JR, Edwards RL, Whalley AJS.
Metabolites of the higher fungi. 22. 2-butyl-3-methylsuccinic acid and 2-hexylidene-3-methylsuccinic acid from Xylariaceous fungi.
J Chem Soc Perk Trans I. 1985 7:1481-6.
Barnes B.
A note on longevity in Xylaria.
Trans Brit Mycol Soc. 1940 24(3/4):356.
Chinworrungsee M, Kittakoop P, Isaka M, Rungrod A, Tanticharoen M, Thebtaranonth Y.
Antimalarial halorosellinic acid from the marine fungus Halorosellinia oceanica.
Bioorg Med Chem Lett. 2001 11(15):1965-9.
Dagne E, Gunatilaka AAL, Asmellash S, Abate D, Kingston DGI, Hofmann GA, Johnson RK.
2 new cytotoxic cytochalasins from Xylaria obovata.
Tetrahedron. 1994 50(19):5615-20.
Fenwick GA.
Xylaria polymorpha in laboratory culture
Mycologist. 1994 8(4) 166-8.
Gunawan S, Steffan B, Steglich W.
Xylaral, a hydroxyphthalide derivative from fruiting bodies of Xylaria polymorpha (Ascomycetes).
Liebigs Annalen Der Chemie. 1990 (8):825-7.
Jang YW, Lee IK, Kim YS, , Lee S, Lee HJ, Yu SH, Yun BS.
Xylarinic acids A and B, new antifungal polypropionates from the fruiting body of Xylaria polymorpha.
J Antibiotics. 2007 60(11):696-9.
Rai BK, Ayachi SS, Rai A.
A note on ethno-myco-medicines from central India
Mycologist. 1993 7(4):192-3.
Rogers JD, Callan BE.
Xylaria polymorpha and its allies in the continental USA.
Mycologia. 1986 78(3):391-400.
1st page from Jstor
Sivanesan A., Holliday P.
Xylaria polymorpha. [Descriptions of Fungi and Bacteria].
IMI Descriptions of Fungi and Bacteria. 1998 36:355.
Snatzke G, Wolff HP.
Mannitol from Xylaria polymorpha.
Zeitschrift für Mykologie. 1987 53(1):137-8.
Yang L, Huaan W.
Cultivation and polysaccharide extraction of Xylaria polymorpha.
Mycosystema. 2004 23(4):536-47.

