The oyster rollrim, Tapinella panuoides.
Synonyms
Agaricus panuoides Fr.
Crepidotus panuoides (Fr.) Pilát
Paxillus fagi Berk. & Broome
Paxillus panuoides (Fr.) Fr.
Paxillus panuoides var. fagi (Berk. & Broome) Cooke
Paxillus panuoides var. ionipes Quél.
Paxillus panuoides var. rubrosquamulosus Svrček & Kubička
Plicaturella panuoides (Fr.) Rauschert
Serpula panuoides (Fr.) Zmitr. ex Zmitr.
Common name
Oyster rollrim
Description
Cap: 2.0-10.0 cm broad, shell or fan-shaped, often shallowly lobed or wavy; margin incurved, decurved at maturity; surface finely hairy when young, and at base of cap, elsewhere matted-tomentose. Color tan to buff-brown, frequently tinged ochre; context pallid, firm when young, flaccid in age, up to 1.0 cm thick, darkening slightly when cut; odor faintly resinous to aromatic; taste not distinctive.
Gills: close, relatively narrow, radiating from a basal attachment point, edges even, appearing wrinkled when viewed from above, especially in age; gills intervenose, occasionally forked, sometimes appearing poroid at maturity, easily separated from the cap context; color cream-buff to dull orange, becoming apricot-buff to warm tan-brown, unchanging when bruised; lamellulae poorly defined.
Stem: absent; adjoining fruiting bodies usually fused at the base; laterally attached to the substrate.
Spores: 4-6 x 3-4 µm, broadly ellipsoid, smooth, thin-walled, hilar appendage not distinct, often dextrinoid in Melzer’s reagent.
Spore print: tan-brown to pale ochraceous-brown.
Habitat: solitary to clustered, often in overlapping shelves on conifer wood, occasionally in wood chips; fruiting late summer to late autumn.

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Bioactive compounds
The literature reveals that this species has been a rich source of novel and interesting compounds. The medicinal potential of these biologicaly active metabolites, although barely explored, is impressive.
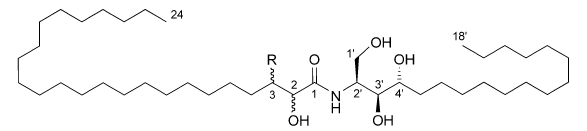
A new phytosphingosine-type ceramide, named paxillamide (aka 2,3-dihydroxy-N-[(1S,2S,3R)-2,3-dihydroxy-1-(hydroxymethyl) heptadecyl]tetracosanamide – see below), was isolated from the CHCl3/MeOH extract of the fruiting bodies of Paxillus panuoides (Gao et al., 2004). Ceramides – currently a hot topic in lipid research – are important in biology because of their involvement in signal transduction pathways and ultimately, in important cellular phenomena like cell growth and apoptosis.
Several steroids have been isolated from Tapinella panuoides, including
- paxillosterone
- panuosterone
- 25-hydroxypanuosterone
- 20-hydroxyecdysone
- ponasterone A
- malacosterone
- turkesterone
The ecdysteroids are structurally related to the insect moulting hormone ecdysone (Vodac et al., 1998). Apparently these ecdysteroids may have taxonomic value, in that they may be used to help identify and distinguish members of the genera Paxillus and Tapinella.
Medicinal properties
Free-radical scavenging activity/neuroprotective effects
Two p-terphenyl compounds, identified as leucomentin-4 and leucomentin-2 were isolated from from methanolic extract of the fruit body of Paxillus panuoides. These compounds strongly inhibited lipid peroxidation in rat liver microsomes with IC50 values of 0.10 and 0.06 µg/ml, respectively (Yun et al., 2000). It was later suggested the neuroprotective effect that the leucomentins have is due to their ability to chelate iron (when present with DNA and H2O2), thus inhibiting iron-mediated oxidative damage to DNA (Lee et al., 2003).
Purified lectins from T. panuoides extracts were able to hemagglutinate human red cells, with an anti-A specificity. The reaction of anti-A agglutinin was strongly inhibited by N-acetyl-D-galactosamine (Furukawa et al., 1995).
Several of the p-terphenyl ortho-quinones described above were found to be active in assays against tumor cell cultures (Cali et al., 2004).
Cali V, Spatafora C, Tringali C.
Sarcodonins and sarcoviolins, bioactive polyhydroxy-p-terphenyl pyrazinediol dioxide conjugates from fruiting bodies of the basidiomycete Sarcodon leucopus.
Eur J Org Chem. 2004 (3):592-9.
Furukawa K, Ying R, Nakajima T, Matsuki T.
Hemagglutinins in fungus extracts and their blood group specificity.
Exp Clin Immunogenet. 1995 12(4):223-31.
Gao JM, Zhang AL, Zhang CL, Liu JK.
Paxillamide: a novel phytosphingosine derivative from the fruiting bodies of Paxillus panuoides.
Helv Chim Acta 2004 87(6):1483-7.
Lee IK, Yun BS, Kim JP, Ryoo IJ, Kim YH, Yoo ID.
Neuroprotective activity of p-terphenyl leucomentins from the mushroom Paxillus panuoides.
Biosci Biotechnol Biochem. 2003 67(8):1813-6.
Vokac K, Budesinsky M, Harmatha J, Kohoutova J.
Ecdysteroid constituents of the mushroom Tapinella panuoides.
Phytochem. 1998 49(7):2109-14.
Yun BS, Lee IK, Kim JP, Yoo ID.
Leucomentin-5 and -6, two new leucomentin derivatives from the mushroom Paxillus panuoides.
J Antibiot (Tokyo). 2000 53(7):711-3. No abstract available.




