The medicinal mushroom Coprinopsis atramentaria The inky cap, Coprinopsis atramentaria, growing on a stump in Elk Island National Park, near Edmonton, Canada.
Classification
Kingdom Fungi
Phylum Basidiomycota
Class Basidiomycetes
Order Agaricales
Family Agaricaceae
Genus Coprinopsis
Synonyms
Agaricus atramentarius Bull.
Agaricus fimetarius sensu Sowerby (1799)
Agaricus luridus Bolton
Agaricus plicatus Pers.
Agaricus sobolifer Hoffmann
Coprinus atramentarius (Bull.) Fr.
Coprinus atramentarius var. soboliferus (Fr.) Rea
Coprinus luridus (Bolton) Fr.
Coprinus plicatus (Pers.) Gray
Coprinus sobolifer Fr.
Common names
Inky cap
Tippler’s bane

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Description
Cap: 2-8 cm diameter, grey to greyish-brown; oval then campanulate or conical; fibrillose; radially grooved almost to disc; margin irregularly notched and lobed, splitting when the cap expands.
Flesh: thin, greyish.
Gills: free, initially white, then black and deliquescing; up to 1 cm broad, cottony on the edge, crowded.
Stem: 10-15 cm long, 8-15 mm thick; equal; silky white to very light brown, with small upward pointing scales below the ring; readily separates from cap.
Spore print: black.
Spores: smooth, ellipsoid, 8-12 x 4.5-6.5 µm.
Habitat and distribution: grows in caespitose clusters or gregariously in fields and gardens, in rich soils or around stumps. Common in the Northern Hemisphere; has been reported in South Africa (Reid and Eicker, 1999).
Edibility: edible with caution – poisonous if consumed with alcohol.
Research has shown that, when grown on a compost bed, this mushroom required a cold shock (in this case, a temperature reduction from 25°C to 20°C) to initiate the formation of fruiting bodies (Stott and Broderick, 1995).
Bioactive compounds
The poisonous effects of C. atramentaria are caused by a molecule named coprine, or N5-1-hydroxycyclopropyl-L-glutamine (shown below), which is metabolized to 1-aminocyclopropanol. This latter compound then inhibits the enzyme alcohol dehydrogenase, which normally oxidizes alcohol (i.e., ethanol) into acetaldehyde, indicated by the reaction below:
CH3CH2OH + NAD+ → CH3CHO + NADH + H+
After alcohol intake under the influence of coprine, the concentration of acetaldehyde in the blood may be 5 to 10 times higher than that found during metabolism of the same amount of alcohol alone. As acetaldehyde is one of the major causes of the symptoms of a “hangover” this produces immediate and severe negative reaction to alcohol intake. Some 5-10 minutes after alcohol intake, the unfortunate victim may experience the effects of a severe hangover for a period of 30 minutes up to several hours. Symptoms include flushing of the skin, accelerated heart rate, shortness of breath, nausea, and vomiting. The ill effects of coprine may be felt if alcohol is consumed for up to several days after eating the mushroom. The biochemical mechanism of action of coprine and the resultant symptoms are similar to the drug disulfiram, used to treat chronic alcoholism (Tottmar and Lindberg, 1977; Carlsson et al., 1978).
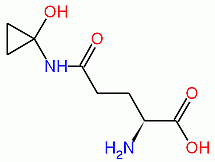
Coprine, ADH-inhibiting metabolite from C. atramentaria.
Coprine was shown to cause testicular lesions in mice and dogs (Jönsson et al., 1979), adding another note of caution to those wishing to consume it.
Medicinal properties
Anti-tumor
Polysaccharides extracted from the mycelial culture of C. atramentaria and administered intraperitoneally into white mice at a dosage of 300 mg/kg inhibited the growth of Sarcoma 180 and Ehrlich solid cancers by 100% (Ohtsuka et al., 1973).
The aqueous extract of Tippler’s bane was shown to reduce the mycelial growth and inhibit sporulation of Penicillium expansum, a pathogenic mold (Florianowicz, 2000).
References
Carlsson, A, Henning, M, Lindberg, P, Martinson, P, Trolin, G, Waldeck, B, Wickberg, B.
Disulfiram-like effect of coprine, pharmacologically active principle of Coprinus atramentarius.
Acta Pharmacologica Et Toxicologica. 1978 42(4):292-7.
Florianowicz T.
Inhibition of growth and sporulation of Penicillium expansum by extracts of selected basidiomycetes.
Acta Societatis Botanicorum Poloniae. 2000 69(4):263-7.
Hatfield GM, Schaumberg JP.
Isolation and structural studies of coprine, disulfiram-like constituent of Coprinus atramentarius.
Lloydia-the Journal of Natural Products. 1975 38(6):489-96.
Jonsson M, Lindquist NG, Ploen L, Ekvarn S, Kronevi T.
Testicular lesions of coprine and benzcoprine.
Toxicology. 1979 12(2):89-100.
Lee IK, Jeong CY, Cho SM, Yun BS, Kim YS, Yu SH, Koshino H, Yoo ID.
Illudins C-2 and C-3, new illudin C derivatives from Coprinus atramentarius ASI20013.
J Antibiotics. 1996 49(8):821-2.
Lindberg, P, Bergman, R, Wickberg, B.
Isolation and structure of coprine, a novel physiologically active cyclopropanone derivative from Coprinus atramentarius and its synthesis via 1-aminocyclopropanol.
J Chem Soc-Chem Comm. 1975 (23):946-7.
Lindberg, P, Bergman, R, Wickberg, B.
Isolation and structure of coprine, in vivo aldehyde dehydrogenase inhibitor in Coprinus atramentarius – syntheses of coprine and related cyclopropanone derivatives.
J Chem Soc-Perk Trans 1. 1977 (6):684-91.
Michelot, D.
Poisoning by Coprinus atramentarius.
Nat Toxins. 1992 1(2):73-80. Review.
Mohamed SH, Dix NJ.
Resource utilization and distribution of Coprinus comatus, Coprinus atramentarius, Lacrimaria velutina and Melanoleuca grammopodia.
Transactions of the British Mycological Society. 1988 90:255-63.
Ohtsuka S, Ueno S, Yoshikumi C, Hirose F, Ohmura Y, Wada T, Fujii T, Takahashi E.
Polysaccharides having an anticarcinogenic effect and a method of producing them from species of Basidiomycetes.
UK Patent 1331513, 26 September 1973.
Reid DA, Eicker A.
South African fungi 10: New species, new records and some new observations.
Mycotaxon. 1999 73:169-97.
Stott K, Broderick A.
Improved fruiting of Coprinus atramentarius using cold-shock treatment during growth.
World J Microbiol Biotechnol. 1995 11(6):693-4.
Tottmar O, Lindberg P.
Effects on rat-liver acetaldehyde dehydrogenases in vitro and in vivo by coprine, disulfiram-like constituent of Coprinus atramentarius.
Acta Pharmacologica Et Toxicologica. 1977 40(4):476-81.





There is a serious error in paragraph1, second sentence, of your section “Bioactive compounds”. The enzyme inhibited is not Alcohol Dehydrogenase, which metabolises ethanol into acetaldehyde but Acetaldehyde Dehydrogenase which facilitates the next step, the metabolism of Acetaldehyde. If the first step is inhibited there would be no acetaldehyde formed. It is the acumulation of acetaldehyde that causes the problems.