The “dung-loving bird’s nest”, Cyathus stercoreus (Schwein.) de Toni. These specimens were found in Japan.
Synonyms
Cyathia stercorea (Schwein.) V.S. White
Bull. Torrey bot. Club 29: 266 (1902)
Nidularia stercorea Schwein.
Trans. Am. phil. Soc. 4(2): 253 (1832)
Common name
Dung loving bird’s nest
Description
Fruiting bodies: up to 1.5 cm tall x 4-8 mm wide, goblet-shaped (obconic) “nests”. The inner surface is nongrooved and nonfurrowed, which helps distinguish it from the related bird’s nest fungi Cyathus striatus. The outer surface is tomentose (more so when young) and yellow-brown in color, becoming smoother and darker in color in maturity. Periodioles are attached to the fruiting bodies by a thin cord called a funiculus. Peridioles are dark grey to black in color, 1-2 mm in diameter.
Spores: variable in size and shape, but typically globose or oval, smooth, thick-walled, and 18-40 x 18-30 µm. Further details about spore morphology may be found in Brodie and Dietrich (1977).
Habitat: this saprobic species is found growing on dung of herbivores, on soil containing manure, or on wood chips. Grows gregariously or in dense clusters.
The ultrastructure of this species has been described in detail (Flegler and Hooper, 1978).
Medicinal properties
Antioxidant effects
The polyketide antioxidative compounds, cyathusals A, B, and C as well as the previously known compound pulvinatal have been isolated from C. stercoreus (Kang et al., 2007). The cyathusals have antioxidant activity (measured by DPPH and ABTS radical scavenging assays) roughly comparable to reference antioxidants Trolox and BHA.
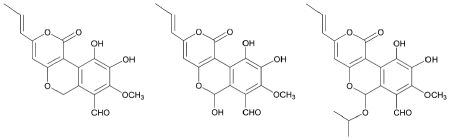
Further investigations led to the discovery of of cyathuscavins A, B, and C along with the known compound 4-hydroxy-6-propenyl-pyran-2-one (Kang et al., 2008). The former 3 compounds showed good antioxidative activity, as measured by radical scavenging activity in the DPPH assay, and the ABTS+ assay. Cyathuscavins A and B (but not C) also scavenged superoxide (O2-) radicals more effectively than the control compound BHA. All three cyathuscavins also protected supercoiled DNA from Fenton reaction-mediated DNA breakage.
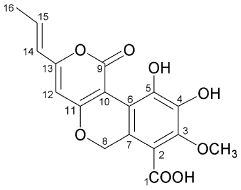
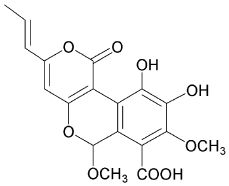
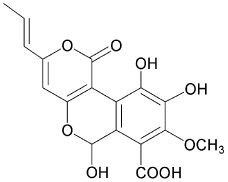
4-hydroxy-6-propenyl-pyran-2-one.

My name is Austin Collins.
I've dedicated my life to Mushrooms.
I believe Mushrooms are the best kept secret when it comes to health and well being.
For that reason, I would like to share a company with you that in my opinion makes the best mushroom products on the market.
The company is called Noomadic Herbals, my favorite supplement they make is called "Mushroom Total".
I take their products every day and they have helped me think better and have more energy. Give them a try.
-Austin
Links
References
Brodie HJ, Dietrich HF.
Spore morphology in the Nidulariaceae fungi as revealed by the scanning electron microscope.
Can J Bot 1977 55(24):3042-3045.
Flegler SL, Hooper GR.
Ultrastructure of Cyathus stercoreus.
Mycologia 1978 70(6):1181-1190.
Kang H-S, Jun E-M, Park S-H, Heo S-J, Lee T-S, Yoo I-D, Kim J-P.
Cyathusals A, B, and C, antioxidants from the fermented mushroom Cyathus stercoreus.
J Nat Prod 2007 70(6):1043-1045.
Kang H-S, Kim K-R, Jun E-M, Park S-H, Lee T-S, Suh J-W, Kim J-P.
Cyathuscavins A, B, and C, new free radical scavengers with DNA protection activity from the Basidiomycete Cyathus stercoreus.
Bioorganic & Medicinal Chemistry Letters. 2008 18(14):4047-4050.



I’m seeing these in my zucchini garden and wondering if they are causing my zucchini plants to die?
They could be. Anything that takes away nutrients from your plants can do that!
How do you prevent and irradiate them? A mushroom killed my dog and I’m joe on a mission to remove all signs and opportunities for them to grow.
I would take away their food source, if that doesn’t work, try a weed killer.
I have this in a pot growing mint, I’m in Phoenix. I don’t know if I should leave because it’s good or take because it’s bad. I don’t see I change in my mint really. I do have a family of stomach pain sufferers lol what should I do!?